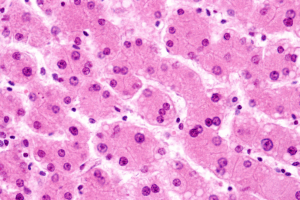
Modeling adaptive immune reactions in vitro is a significant gap in liver research and drug development. Immune-mediated liver injuries like drug hypersensitivity, autoimmune hepatitis, and immune-related adverse effects from cancer immunotherapy are caused by complicated and patient-specific mechanisms. Current preclinical processes cannot be reproduced. Idiosyncratic drug-induced liver injury (iDILI) represents a major clinical and regulatory concern, as it is a leading cause of drug withdrawals and safety warnings. It also accounts for a significant proportion of acute liver failure cases unrelated to overdose.
Current in vitro methods can not mimic the Human Leukocyte Antigen (HLA)-restricted, cytotoxic CD8⁺ T-cell responses that mediate the immune-specific hepatocyte injury that leads to translational gap stems. Advances like induced pluripotent stem cell (iPSC)-derived hepatocytes and the liver-on-chip method have enhanced the physiological fidelity of hepatic metabolism and innate immune signaling. However, it still fails to replicate the adaptive immune pathways that are crucial for accurately modeling immune-mediated hepatotoxicity.
The aim of this study was to develop and validate a fully human and matrix-free liver organoid (HLO) microarray platform, which integrated iPSC-derived hepatocytes with the autologous CD8⁺ T cells in a genetically defined context. It also established a scalable and reproducible co-culture system, which is capable of modeling the antigen-specific immune reaction and immune-mediated hepatotoxicity. It bridges the gap in functional immune response and genetic susceptibility in iDILI.
Researchers developed a matrix-free HLO microarray by using iPSC-derived hepatocytes co-cultured with autologous CD8⁺ T lymphocytes from donors with specific HLA genotypes. This system minimizes the dependence on primary cells and Matrigel scaffolds, thereby improving standardization and scalability without compromising physiological relevance. The model was tested by using flucloxacillin. It is well known that antibiotics linked with HLA-B57:01 are associated with T-cell-mediated hepatotoxicity. Only a subset of HLA-B57:01 carriers develop liver injury, which makes flucloxacillin a proper target to test whether the model could reproduce the donor-specific immune activation. Researchers analyzed cytokine secretion, CD8⁺ T-cell activation, and hepatocyte apoptosis to assess the immune response dynamics and hepatotoxic results.
The HLO microarray platform accurately modeled antigen-specific immune reactions linked to flucloxacillin-induced hepatotoxicity. The co-culture system showed robust CD8⁺ T-cell activation and cytokine release after drug administration. It aligns with the known immunopathogenic mechanism of iDILI. Hepatocyte apoptosis was in an HLA-B*57:01-dependent manner and mimics the in vivo immune-mediated injury seen in patients. The platform replicated key hallmarks of adaptive immune activation, like T-cell priming, antigen representation, and effector cytotoxicity, without the necessity of supra-physiological stimulation or animal systems. Traditional hepatotoxicity assays, which rely on direct cytotoxic measures and depend on precise characterization of immune-driven hepatocyte injury. The system demonstrated reproducibility across multiple donors and holds promise for investigating inter-individual variability in immune responses.
This study introduces a novel, fully human and matrix-free liver organoid microarray platform, which accurately models adaptive immune-mediated hepatotoxicity.
The study presents a novel, human, and matrix-free liver organoid microarray technology that properly simulates adaptive immune-mediated hepatotoxicity. This approach combines iPSC-derived hepatocytes with autologous CD8⁺ T cells in a genetically controlled milieu, which bridges the gap between genetic propensity and functional immunological responses. The platform’s effective recapitulation of HLA-B*57:01-linked flucloxacillin hepatotoxicity demonstrates its use as a preclinical tool for evaluating immune-related medication safety. It solves constraints of previous models and allows for scaled, reproducible testing. The platform can be used to study the autoimmune liver diseases and immunotherapy-induced hepatotoxicity.
Reference: Soussi FEA, Brusilovsky M, Buck E, et al. Autologous organoid–T cell co-culture platform for modeling of immune-mediated drug-induced liver injury. Adv Sci. 2025; e08584. doi:10.1002/advs.202508584