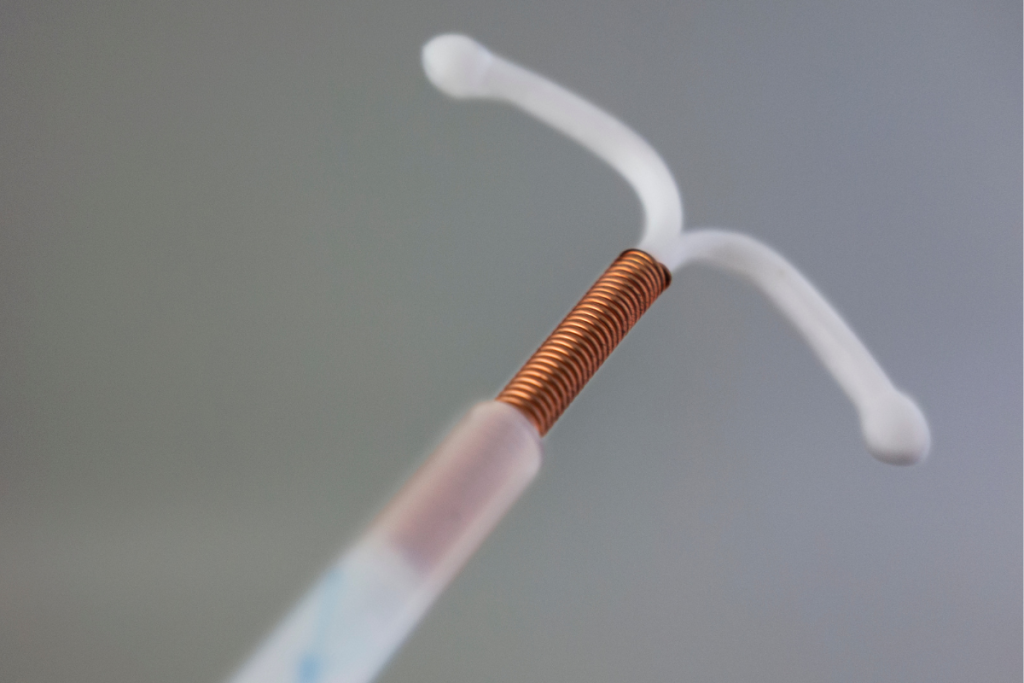
MIUDELLA® (copper) received FDA approval as a breakthrough copper intrauterine system from Sebela Women’s Health Inc., a subsidiary of Sebela Pharmaceuticals, in Roswell, GA, on February 25, 2025. In the United States, this is the first hormone-free intrauterine device (IUD) approved for pregnancy prevention for more than four decades. For women of reproductive age, this drug provides a hormone-free contraceptive option for birth control. With advanced technology, the next-generation IUD has been designed to offer long-acting contraception for three years with lower concentrations of copper compared to currently available copper-based devices. It features a nitinol frame with a flexible design and streamlined techniques for insertion.
Dr. Kelly Culwell from Sebela Women’s Health Inc. emphasized the innovation and stated, “The new IUD effectively prevents pregnancies using less than half the copper of existing U.S. copper-based IUDs. It aims to overcome barriers to use and provide a hormone-free option for women.” She also highlighted that the latest advances would enhance user experience and improve adoption.
The long-acting reversible contraception (LARC) method is recommended by the American College of Obstetricians and Gynecologists (ACOG) as a highly effective option with minimal contraindications and shows better management to the various patient groups. Having a wide range of available birth control methods, 41.6% of pregnancies in the U.S. are still classified as unplanned.
Patients have access to the smallest hormone-free IUD available in the U.S., which measures 32 mm by 30 mm. This device inserter includes a customized loading system, a cylindrical and tapered tube with a narrow 3.7 mm diameter tip, and a rounded blade for simplified placement.
Dr. David K. Turok, professor and principal investigator in the Department of Obstetrics and Gynecology at the University of Utah, stated that it has been four decades since a new hormone-free IUD option became available to women. So, I’m thrilled by the clinical data demonstrating the efficacy and safety of MIUDELLA®. This will potentially improve continuation rates in reducing pain and bleeding with decreased expulsion rates for those seeking non-hormonal options.
MIUDELLA was evaluated in three clinical trials involving 1,904 women aged 17 to 45 at 42 sites. Contraceptive efficacy was evaluated over three years using the Pearl Index in the Phase 3 study, with a first-year value of 0.94 and a cumulative three-year value of 1.05, which indicates 99% effectiveness. The system also recorded a high placement success rate of 98.8%, with users and clinicians providing positive experiences and feedback. The common side effects included heavy menstrual bleeding, dysmenorrhea, and intermenstrual bleeding, but their prevalence lessened over the course of time. The discontinuation rate because of these side effects declined from 8.5% in 1st year to 3.2% in 3rd year, and the expulsion rates remained low, ranging from 1.9% in 1st year to 0.9% in 3rd year.
It is distributed exclusively to trained healthcare providers as part of a Risk Evaluation and Mitigation Strategy (REMS) program to ensure appropriate use. Insertion training becomes a mandatory requirement for healthcare providers who will act as first-time device distributors. It will be commercially available to patients through medical specialists who became certified in 2025.
References: Sebela Pharmaceuticals Inc. FDA approves MIUDELLA®, the first hormone-free copper intrauterine system (IUS) in the U.S. in over 40 years, from Sebela Women’s Health Inc. PR Newswire. Published February 25, 2025. https://www.prnewswire.com/news-releases/fda-approves-miudella-the-first-hormone-free-copper-intrauterine-system-ius-in-the-us-in-over-40-years-from-sebela-womens-health-inc-302067256.html