Background
HSCT comprises a therapy that utilizes stem cells to restore many types of immune system disorders, hematology diseases, and certain types of cancer. The HSCT process entails transferring hematopoietic stem cells, precursor cells that develop into several blood cell types, such as the white blood cells, the red blood cells, and the platelets. HSCT is a complex and often very critical medical treatment that seeks to transplant and correct a patient’s impaired or nonworking hematopoietic system. The treatment is long and has undergone changes to its method over the years, but it is now a standard procedure for many conditions.
The history of HSCT was pioneered in the middle of the 20th century based on hematopoiesis experiments and observations, which focused on the bone marrow.
E. Donnall Thomas and his group performed the initial clinical BMT in 1956.
Indications
Leukemias: Standard HSCT is applied for the treatment of acute leukemias such as acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). It may be advisable for relapse high risk patients or who do not respond to conventional treatment regimens for complete remission.
Multiple Myeloma: It is used in multiple myeloma, a disease that affects the plasma cells. In this situation autologous stem cell transplantation ofstem cells are harvested from the patient is another treatment modality for suitable patients.
Myelodysplastic Syndromes (MDS): MDS is a type of cancer commonly referred to as aplastic anemia because it involves the abnormal formation of blood cells in the body. HSCT can be performed on patients with higher-risk MDS who have not improved with other treatments.
Primary Immunodeficiency Disorders: SCID and other PID patients use HSCT to build a proper immune system since they are born with nonfunctioning immune cells.
Hemoglobinopathies: Other hemoglobinopathies that may be offered HSCT include those where symptoms are severe, and no other management modality exists.
Contraindications
Uncontrolled Infections: Due to the extent of active, uncontrolled infections that are associated with complications of HSCT during and after transplant, the patient with chronic infections may need to wait until their infection is controlled before they can be considered for transplantation.
Organ Dysfunction: Patients with compromised cardiac and pulmonary function, hepatic or renal diseases, may not be suitable candidates for HSCT. There are also certain preconditions that may considerably complicate the transplantation process and possibly increase the risks that are inherent in it.
Advanced Age: Age itself is not a specific contraindication, but people of the advanced age may likely become more prone to complications. In older individuals, general health and fitness are generally the deciding factors for deciding whether to proceed with HSCT.
Active Bleeding Disorders: Some patients with bleeding disorders who are currently having active bleeding or at high risk of bleeding should not undergo HSCT. This process requires myeloablative conditioning regimens that used to significantly cause reductions in blood cell densities.
Outcomes
Overall Survival: In HSCT, the primary graft outcome measures are frequent relief of symptoms and overall survival. This shows the overall disease specific survival, graft survivals, and rejection rates depending on the disease, type of transplant, and other considerations. This type of transplants may be curative in some of the hematologic malignancies hence making them a suitable option.
Disease Remission: For diseases such as leukemia and lymphoma, complete remission of the disease is a positive result. HSCT is most utilized for patients in remission, though more therapy or a relapse may be required.
Quality of Life: Patients need long periods to recover after receiving transplants and may experience complications such as graft dysfunction or rejection, fatigue, psychosocial complications, and other issues.
Autologous Transplants: When the stem cells utilized are from the patient’s own body, the risks of GVHD are considerably lower than in other types of transplants but may present a set of other problems. This medication is used in the treatment of diseases as Multiple Myeloma and Lymphomas.
Equipment
Apheresis Machine
Stem Cell Cryopreservation System
Biological Safety Cabinets (BSC)
Incubators and CO2 Chambers
Laminar Flow Hoods
Infusion Pump
Central Venous Catheters
Leukapheresis Catheters
IV Poles and Tubing
Sterilization Equipment
Radiation Therapy Equipment
Pharmacy Equipment
Monitoring and Support Devices
Patient preparation
Donor Sources:
Autologous Transplantation: The patient serves a donor and stem cells can be harvested from the patient’s bone marrow or from the patient’s circulating blood.
Allogeneic Transplantation: These stem cells are collected from a matching donor. It can be matched (identical), which means the donor and recipient are at least partially genetically similar, or it can be a mismatched transplant depending on the protocol.
Donor Selection: Especially for allogeneic transplants, donor selection remains critical in determining a favourable outcome. Factors considered include:
HLA Matching: Human leukocyte antigen (HLA) compatibility between the donor and the recipient is a crucial factor. Substantial evidence exists that indicates the recipient and donor match significantly influences the occurrence of GVHD, the lower the match, the higher the risk of developing this condition.
Age and Health: There are additional factors that can influence the general health state of the donor, the age of the donor and if the later has any serious illness that may affect the quality of the donated blood.
Stem Cell Source: Sources such as bone marrow, umbilical cord blood or peripheral blood may be selected based on the availability of the donors and the specific protocol to be followed.
Hematopoietic Stem Cell Transplantation
Step-1: Conditioning Regimen: As a preliminary step, the patients receive a conditioning regimen before receiving the stem cells. This may be combined with intense chemotherapy regimens and, in specific scenarios, total body irradiation (TBI). The intention is to remove the recipient’s own bone marrow, weaken their immune system, and make space for the new stem cells.
Step-2: Stem Cell Infusion: After the pre-transplantation phase, the final part and the core of the transplantation process comes with the stem cells administration. The stem cells can be collected from different sources which include; Bone Marrow Stem Cells, Peripheral Blood Stem Cells and, Umbilical Cord Stem Cells.
Autologous Transplantation: In autologous transplants stem cells are retrieved from the patient prior to the onset of the conditioning protocol. After conditioning, the patient’s own stem cells, which have been collected and preserved (cryopreserved) previously, are reintroduced into the patient’s circulation through a process called stem cell transplant.
Allogeneic Transplantation: In allogeneic transplants the stem cells are harvested from the donor and are then transfused into patient’s circulating blood through a central venous catheter. This process is like sending in the artificial blood wherein the stem cells gradually circulate in the person’s body.
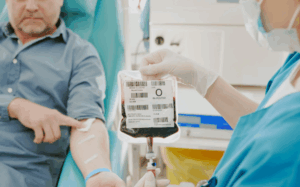
Hematopoietic Stem Cell Transplantation
Umbilical Cord Blood Transplantation: In umbilical cord blood transplantation, the blood that was processed and stored is placed in a frozen state and then infused into the patient.
Step-3: Further Support: Patients with compromised stem cells are closely observed for such adverse effects. This includes monitoring of physical stability through regular checking of their basic vital parameters, blood samples to check counts, and general signs of engraftment.
Step-4: Engraftment: It is a very crucial situation where the infused stem cells find their way to the bone marrow and start organizing a new functional hematopoietic system. This phase involves several key steps:
This phase involves several key steps:
Migration to Bone Marrow: The injected stem cells enter the circulatory system and then migrate to the stem cell niche of bones. Chemokines and other signaling molecules direct this movement.
Engraftment and Colonization: Stem cells that are infused into patients circulate in the peripheral blood system and then to the bone marrow niches where they interact with the host stromal cells to start the engraftment process. This is when the stem cells of the embryo start to develop into mature blood cells, including the WBCs, RBCs, and platelets.
Neutrophil Engraftment: Neutrophils are the first to return when engraftment is successful This is true because certain elements point to successful engraftment when they return to normal. This was because of the ability that the immune system of the body was now being reestablished at this age.
Platelet and Red Blood Cell Engraftment: While the neutrophil count recovers quickly after engraftment, platelet and red blood cell counts also return gradually. This process may take some weeks and often the patients will need transfusion with blood products.
Step-4: Post-Engraftment Care: Post-engraftment comes next, and the patients require medical attention throughout their post-transplant period. It includes a regular check-up, provision of comfort, and measures taken to prevent the spread of ailments.
Step-6: Recovery of the immune system: This recovery of immunity improvement is gradual process. It poses a risk for patients because they are more susceptible to infections. The prophylactic antibiotics and antifungal medications may be prescribed Thus, antibiotics may be prescribed, antifungal should also be taken in use
Step-7: Graft-versus-Host Disease (GVHD) Monitoring: This is a widely observed form of toxicity, particularly in allogeneic transplants where donor’s immune cells can target host tissues. The main side effects are that patients are watched for signs of GVHD, and immunosuppressive drugs are given if necessary.
Step-8: Long term management: This entails follow up of patient outcomes, frequent medical check-ups, follow up for risk outcomes and side effects. It is also important to ensure long-term care that includes certain psychological and social care requirements.
Complications
Graft-versus-Host Disease (GVHD): A significant issue associated with the allogeneic transplants is graft versus host disease where the donor’s immune cells transfer to the recipient and attack its tissues.
Infections: This is especially attributed by the fact that during the preparative phase of the HSCT, the immune system of the patient is eliminated or reduced thus increasing the susceptibility to infections. Infections may include the bacterial, fungal, viral, or parasitic type. Such patients may have to be given some prophylactic antibiotics and antiviral drugs, and isolation measures might be necessary for such patients.
Engraftment Syndrome: Engraftment syndrome is cluster of various symptoms that can develop after stem cell infusion includes fever, rash, pulmonary congestion, liver dysfunction. This is thought to be related to the early setting of the graft, and the production of cytokines, as well as other conditions.
Pulmonary Complications: Hematologic complications such as anemia, leukopenia, and thrombocytopenia are also possible, as is respiratory involvement in the form of pneumonia, ARDS, and interstitial pneumonitis. A measure of the respiratory symptoms and timely intervention is critically important to avoid adverse pulmonary complications.
Venous Complications: This Utilized for stem cell infusion and continuative care, can stimulate adverse effects regarding veins, such as infections, thrombosis, and catheter-related bloodstream infections. It is imperative that proper care and observation of the catheter is provided to a patient since it can lead to a host of complications.
Secondary Malignancies: There is more likelihood, in patients who have undergone HSCT, developing secondary cancers like leukemia or solid tissue tumors later in life. It is important to perform screening tests and assessment of later effects for these complications in survivors.
References

HSCT comprises a therapy that utilizes stem cells to restore many types of immune system disorders, hematology diseases, and certain types of cancer. The HSCT process entails transferring hematopoietic stem cells, precursor cells that develop into several blood cell types, such as the white blood cells, the red blood cells, and the platelets. HSCT is a complex and often very critical medical treatment that seeks to transplant and correct a patient’s impaired or nonworking hematopoietic system. The treatment is long and has undergone changes to its method over the years, but it is now a standard procedure for many conditions.
The history of HSCT was pioneered in the middle of the 20th century based on hematopoiesis experiments and observations, which focused on the bone marrow.
E. Donnall Thomas and his group performed the initial clinical BMT in 1956.
Leukemias: Standard HSCT is applied for the treatment of acute leukemias such as acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). It may be advisable for relapse high risk patients or who do not respond to conventional treatment regimens for complete remission.
Multiple Myeloma: It is used in multiple myeloma, a disease that affects the plasma cells. In this situation autologous stem cell transplantation ofstem cells are harvested from the patient is another treatment modality for suitable patients.
Myelodysplastic Syndromes (MDS): MDS is a type of cancer commonly referred to as aplastic anemia because it involves the abnormal formation of blood cells in the body. HSCT can be performed on patients with higher-risk MDS who have not improved with other treatments.
Primary Immunodeficiency Disorders: SCID and other PID patients use HSCT to build a proper immune system since they are born with nonfunctioning immune cells.
Hemoglobinopathies: Other hemoglobinopathies that may be offered HSCT include those where symptoms are severe, and no other management modality exists.
Uncontrolled Infections: Due to the extent of active, uncontrolled infections that are associated with complications of HSCT during and after transplant, the patient with chronic infections may need to wait until their infection is controlled before they can be considered for transplantation.
Organ Dysfunction: Patients with compromised cardiac and pulmonary function, hepatic or renal diseases, may not be suitable candidates for HSCT. There are also certain preconditions that may considerably complicate the transplantation process and possibly increase the risks that are inherent in it.
Advanced Age: Age itself is not a specific contraindication, but people of the advanced age may likely become more prone to complications. In older individuals, general health and fitness are generally the deciding factors for deciding whether to proceed with HSCT.
Active Bleeding Disorders: Some patients with bleeding disorders who are currently having active bleeding or at high risk of bleeding should not undergo HSCT. This process requires myeloablative conditioning regimens that used to significantly cause reductions in blood cell densities.
Overall Survival: In HSCT, the primary graft outcome measures are frequent relief of symptoms and overall survival. This shows the overall disease specific survival, graft survivals, and rejection rates depending on the disease, type of transplant, and other considerations. This type of transplants may be curative in some of the hematologic malignancies hence making them a suitable option.
Disease Remission: For diseases such as leukemia and lymphoma, complete remission of the disease is a positive result. HSCT is most utilized for patients in remission, though more therapy or a relapse may be required.
Quality of Life: Patients need long periods to recover after receiving transplants and may experience complications such as graft dysfunction or rejection, fatigue, psychosocial complications, and other issues.
Autologous Transplants: When the stem cells utilized are from the patient’s own body, the risks of GVHD are considerably lower than in other types of transplants but may present a set of other problems. This medication is used in the treatment of diseases as Multiple Myeloma and Lymphomas.
Apheresis Machine
Stem Cell Cryopreservation System
Biological Safety Cabinets (BSC)
Incubators and CO2 Chambers
Laminar Flow Hoods
Infusion Pump
Central Venous Catheters
Leukapheresis Catheters
IV Poles and Tubing
Sterilization Equipment
Radiation Therapy Equipment
Pharmacy Equipment
Monitoring and Support Devices
Donor Sources:
Autologous Transplantation: The patient serves a donor and stem cells can be harvested from the patient’s bone marrow or from the patient’s circulating blood.
Allogeneic Transplantation: These stem cells are collected from a matching donor. It can be matched (identical), which means the donor and recipient are at least partially genetically similar, or it can be a mismatched transplant depending on the protocol.
Donor Selection: Especially for allogeneic transplants, donor selection remains critical in determining a favourable outcome. Factors considered include:
HLA Matching: Human leukocyte antigen (HLA) compatibility between the donor and the recipient is a crucial factor. Substantial evidence exists that indicates the recipient and donor match significantly influences the occurrence of GVHD, the lower the match, the higher the risk of developing this condition.
Age and Health: There are additional factors that can influence the general health state of the donor, the age of the donor and if the later has any serious illness that may affect the quality of the donated blood.
Stem Cell Source: Sources such as bone marrow, umbilical cord blood or peripheral blood may be selected based on the availability of the donors and the specific protocol to be followed.
Step-1: Conditioning Regimen: As a preliminary step, the patients receive a conditioning regimen before receiving the stem cells. This may be combined with intense chemotherapy regimens and, in specific scenarios, total body irradiation (TBI). The intention is to remove the recipient’s own bone marrow, weaken their immune system, and make space for the new stem cells.
Step-2: Stem Cell Infusion: After the pre-transplantation phase, the final part and the core of the transplantation process comes with the stem cells administration. The stem cells can be collected from different sources which include; Bone Marrow Stem Cells, Peripheral Blood Stem Cells and, Umbilical Cord Stem Cells.
Autologous Transplantation: In autologous transplants stem cells are retrieved from the patient prior to the onset of the conditioning protocol. After conditioning, the patient’s own stem cells, which have been collected and preserved (cryopreserved) previously, are reintroduced into the patient’s circulation through a process called stem cell transplant.
Allogeneic Transplantation: In allogeneic transplants the stem cells are harvested from the donor and are then transfused into patient’s circulating blood through a central venous catheter. This process is like sending in the artificial blood wherein the stem cells gradually circulate in the person’s body.

Hematopoietic Stem Cell Transplantation
Umbilical Cord Blood Transplantation: In umbilical cord blood transplantation, the blood that was processed and stored is placed in a frozen state and then infused into the patient.
Step-3: Further Support: Patients with compromised stem cells are closely observed for such adverse effects. This includes monitoring of physical stability through regular checking of their basic vital parameters, blood samples to check counts, and general signs of engraftment.
Step-4: Engraftment: It is a very crucial situation where the infused stem cells find their way to the bone marrow and start organizing a new functional hematopoietic system. This phase involves several key steps:
This phase involves several key steps:
Migration to Bone Marrow: The injected stem cells enter the circulatory system and then migrate to the stem cell niche of bones. Chemokines and other signaling molecules direct this movement.
Engraftment and Colonization: Stem cells that are infused into patients circulate in the peripheral blood system and then to the bone marrow niches where they interact with the host stromal cells to start the engraftment process. This is when the stem cells of the embryo start to develop into mature blood cells, including the WBCs, RBCs, and platelets.
Neutrophil Engraftment: Neutrophils are the first to return when engraftment is successful This is true because certain elements point to successful engraftment when they return to normal. This was because of the ability that the immune system of the body was now being reestablished at this age.
Platelet and Red Blood Cell Engraftment: While the neutrophil count recovers quickly after engraftment, platelet and red blood cell counts also return gradually. This process may take some weeks and often the patients will need transfusion with blood products.
Step-4: Post-Engraftment Care: Post-engraftment comes next, and the patients require medical attention throughout their post-transplant period. It includes a regular check-up, provision of comfort, and measures taken to prevent the spread of ailments.
Step-6: Recovery of the immune system: This recovery of immunity improvement is gradual process. It poses a risk for patients because they are more susceptible to infections. The prophylactic antibiotics and antifungal medications may be prescribed Thus, antibiotics may be prescribed, antifungal should also be taken in use
Step-7: Graft-versus-Host Disease (GVHD) Monitoring: This is a widely observed form of toxicity, particularly in allogeneic transplants where donor’s immune cells can target host tissues. The main side effects are that patients are watched for signs of GVHD, and immunosuppressive drugs are given if necessary.
Step-8: Long term management: This entails follow up of patient outcomes, frequent medical check-ups, follow up for risk outcomes and side effects. It is also important to ensure long-term care that includes certain psychological and social care requirements.
Graft-versus-Host Disease (GVHD): A significant issue associated with the allogeneic transplants is graft versus host disease where the donor’s immune cells transfer to the recipient and attack its tissues.
Infections: This is especially attributed by the fact that during the preparative phase of the HSCT, the immune system of the patient is eliminated or reduced thus increasing the susceptibility to infections. Infections may include the bacterial, fungal, viral, or parasitic type. Such patients may have to be given some prophylactic antibiotics and antiviral drugs, and isolation measures might be necessary for such patients.
Engraftment Syndrome: Engraftment syndrome is cluster of various symptoms that can develop after stem cell infusion includes fever, rash, pulmonary congestion, liver dysfunction. This is thought to be related to the early setting of the graft, and the production of cytokines, as well as other conditions.
Pulmonary Complications: Hematologic complications such as anemia, leukopenia, and thrombocytopenia are also possible, as is respiratory involvement in the form of pneumonia, ARDS, and interstitial pneumonitis. A measure of the respiratory symptoms and timely intervention is critically important to avoid adverse pulmonary complications.
Venous Complications: This Utilized for stem cell infusion and continuative care, can stimulate adverse effects regarding veins, such as infections, thrombosis, and catheter-related bloodstream infections. It is imperative that proper care and observation of the catheter is provided to a patient since it can lead to a host of complications.
Secondary Malignancies: There is more likelihood, in patients who have undergone HSCT, developing secondary cancers like leukemia or solid tissue tumors later in life. It is important to perform screening tests and assessment of later effects for these complications in survivors.

Both our subscription plans include Free CME/CPD AMA PRA Category 1 credits.

On course completion, you will receive a full-sized presentation quality digital certificate.
A dynamic medical simulation platform designed to train healthcare professionals and students to effectively run code situations through an immersive hands-on experience in a live, interactive 3D environment.

When you have your licenses, certificates and CMEs in one place, it's easier to track your career growth. You can easily share these with hospitals as well, using your medtigo app.



