Background
A left ventricular assist device is a mechanical pump that is surgically inserted into the patient’s chest to help the heart pump blood. People with severe heart failure, whose hearts are unable to pump blood efficiently on their own are the main users of this medical equipment. For those who are not suitable for heart transplantation, LVADs are frequently seen as a bridge to heart transplantation or in some circumstances as destination therapy.
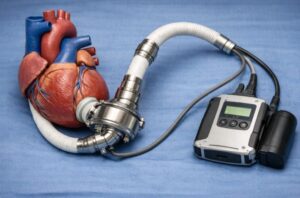
Left ventricular assist device
LVAD is primarily responsible to support the left ventricle, which transports the blood saturated with oxygen from the lungs to the other organs of the body. When heart failure or other conditions damage the heart, LVAD helps to keep the blood flow and enhances the cardiac function.
Indications
Severe Heart Failure: Patients who have severe heart failure who are not improving with traditional treatments are treated with LVADs.
Bridge to Transplant: By preserving the circulation and enhancing health, LVADs give individuals to wait for the heart transplantation with short-term support.
Bridge to Recovery: LVADs can be used to help the heart recover from reversible conditions like post-cardiotomy shock or myocarditis.
Post-Cardiotomy Shock: After heart surgery, LVADs help patients who are experiencing circulatory failure.
Inotrope-Dependent Heart Failure: Patients who need intravenous inotropic drugs are evaluated for LVADs.
End-Stage Heart Failure: Patients without mechanical circulatory assistance who have a reduced life expectancy, LVADs can be used for those.
Contraindications
Irreversible end-organ failure: Patients who have severe heart failure who are still eligible for heart transplantation typically undergo assessments for LVADs. The advantages of LVAD support can be limited in patients who have permanent organ failure.
Active infection: If a patient has an active infection, they may be more susceptible to problems with LVAD implementation. Infection can increase the risk of infections associated with devices and cause systemic problems.
Unresolved bleeding disorders: Patients who have coagulation diseases or other bleeding risk may be more susceptible to bleeding problems if they use LVADs.
Severe right ventricular dysfunction: An LVAD can make severe dysfunction in the right ventricular. Other remedies can be useful and taken into consideration in that condition.
Advanced pulmonary disease: LVADs mainly support the left ventricle. It is not effectively address the main cause of pulmonary difficulties. Patient who has severe pulmonary disease may not be suitable for the LVADs.
Outcomes
Equipment
Pump Unit: The pump is the LVAD’s fundamental component. It supports the heart to pump the blood. There are many types of LVADs like centrifugal and axial flow pumps.
Power Source: LVADs needs an external power source. The patient is connected to an external controller and power pack. Some new device has full implantable system which does not need any external power source.
Controller: The external controller manages and monitors the LVAD’s function. It is a small, portable device that patients carry with them. The controller adjusts the pump speed based on the patient’s activity level and physiological needs.
Power Pack: The power pack is a portable battery that provides power to the LVAD when the patient is away from a direct power source. Patients has spare batteries to make sure the continuous power supply.
Drive Line: The drive line is a cable that connects the implanted pump to the external components, i.e., the controller and power pack. It passes through the patient’s skin, to avoid infections, adequate upkeep and care are essential.
Surgical Driveline Exit Site: This is the area where the drive line exits the patient’s body. It requires regular cleaning and dressing changes to minimize the risk of infection.
Patient preparation
It is essential to obtain a complete medical history like previous cardiac events, surgical operations, and pre-existing health issues. The examination method includes determining the severity of heart failure, assessing ventricular function, and finding any further cardiovascular issues. It is essential to make sure that the patient is provided suitable pharmacological drugs to improve the cardiac function and reduce challenges during and after surgery. Formulate an anticoagulant management plan is necessary to prevent thrombus development in the device. It is necessary to provide detailed information on the Left Ventricular Assist Device (LVAD) like the primary purpose, potential side effects, and the need of maintaining the postoperative care guidelines.
It is important that the patient and their family be aware of both the risks and the benefits of the LVAD installation. Before the surgery, efforts must be made to improve the patient’s physiological status. To reduce perioperative risks, it is important to manage comorbidities like diabetes and hypertension.
Types of LVADs
Pulsatile Flow LVADs: These devices mimic the natural pulsatile motion of heart. It creates a rhythmic activity with each beat. Earlier types of LVADs usually used pulsatile flow.
Continuous Flow LVADs: A large percentage of modern LVADs use continuous flow technology. The blood is continuously pumped by the device. These devices are more compact, durable, and have fewer moving parts that reduces issues.
Axial flow LVADs: It has a single impeller that spins in line with the blood flow axis. These devices have a more streamlined construction and simpler design than other varieties.
Centrifugal Flow LVAD: It uses a spinning impeller to move blood away from the center of rotation. They are known to improve the blood flow and reduce the risk of thrombus development.
Implantable LVADs: These devices are fully implanted into the body, with a driveline which connect the equipment to an external power source and control unit. It is used for long support.
Biventricular help Devices: Other LVADs gives support to the left ventricle. BiVADs gives support to both left and right ventricles. It is used in cases where both chambers fail.
Techniques & approaches
Step 1 The widely recognized method for implementing a durable LVAD entails conducting the implantation while the heart is in operation, utilizing cardiopulmonary bypass.
Step 2 The surgeries involving the implants are conducted using a non-beating heart, cardioplegia & aortic cross-clamp. Additionally, procedures have been carried out both with & without utilization of cardiopulmonary bypass, employing fibrillatory arrest when necessary.
Step 3 Two similar versions of the necessary steps—cutting than sewing or sewing then cutting—can be used to secure the inflow cannula to the left ventricular apex.
Step 4: Using a method of cutting and then sewing, the left ventricular apex undergoes coring. Both procedures are conducted following the administration of suitable anticoagulation.
Step 5 The preference of implanters & the integrity of a heart muscle quality will determine whether to use pledgetted sutures or, interrupted sutures, or running sutures to bind an inflow graft to the left ventricular apex.
Step 6 In the method of sewing before cutting, the inflow graft is firmly attached to the left ventricular apex using the preferred methods & sutures and, subsequently, cored the left ventricular apex.
Step 7 After completing the two processes and excising the left ventricular wall and any papillary muscle from the inflow, the pump is attached to the inflow-sewing ring and securely fastened using the appropriate technique for the pump approach.
Step 8: The aorta is connected to the outflow graft. Usually linked to the ascending aorta on the greater curvature, this is located slightly away from the junction of the sinotubule.
Step 10 The connection can frequently be established using a partial occlusion clamp following the administration of suitable anticoagulants.
Step 11 After a sharp aortotomy, the tiny gauge (5-0,4-0) monofilament suture nonabsorbable is used to sew an outflow graft. Before tying, it is generally beneficial to tighten the suture with nerve hooks.
Step 12 For individuals with an ascending aorta that is calcified rich or those opting for another method, low invasive implant methods, it is possible to attach the outflow graft to a descending thoracic aorta.
Step 13 Using a method like an apical-aortic conduit, centrifugal pumps seem to offer greater adaptability for other implantation strategies.
Step 14 A usual tunnel to the mid-clavicular line is located two to three finger breaths beneath a coastal margin, carrying drivelines that supply power, control, pump & communication.
Step 15 The dermal layer is brought back together, & the driveline is fastened using monofilament suture, which is intended to stay in place till maximum ingrowth occurs in the driveline coating, usually made of velour.
Step 16 With growing frequency, there is a rising trend in adopting alternative implant techniques. These methods may be employed to reduce surgical stress or in response to a challenging mediastinum in individuals undergoing multiple surgical processes.
Laboratory tests
Complete Blood Count: This test helps monitor for anemia, leukopenia, and thrombocytopenia, which are potential complications associated with LVADs.
Electrolyte Panel: Sodium, Potassium, Chloride: Monitoring electrolyte balance is crucial, especially considering the impact of medications and diuretics on these levels.
Inflammatory Markers: Increased level of C-reactive protein, erythrocyte sedimentation rate may indicate the inflammation or infection that affect the LVAD function.
Complications
Hemorrhage: Patients who used LVADs need to administer anticoagulant therapy to mitigate the formation of thrombi which can lead to increased propensity for hemorrhagic incidents. This can lead to gastrointestinal hemorrhage, intracranial hemorrhage, or bleeding at the surgical incision sites.
Infectious Complications: The potential for infections exists at both the implantation site of the LVAD and in the device itself. Sepsis is one of the worst side effects that these diseases can cause.
Thromboembolic Events: Even when anticoagulation procedures are followed, thrombi may still form in the LVAD. This can lead to cerebrovascular accidents or device malfunction.
Mechanical Dysfunction: Mechanical devices are prone to operational failures because of their intrinsic nature. This can lead to complications like electrical or pump breakdowns. To identify and address these problems as soon as possible, routine observation is essential.
Right Ventricular Insufficiency: While LVADs mainly support left ventricular function, there is some possibility for the right heart to deteriorate over time. This condition may lead to failure of right ventricular which needs medical interventions.
Arrhythmogenic Events: The presence of LVADs may cause arrhythmias. Such arrhythmias in people with LVADs may cause significant clinical problems.
Anticoagulants
Warfarin: It helps to prevent the blood clots. Regularly monitor the international normalized ratio is needed.
Antiplatelet Agents:
Aspirin: Often used in conjunction with anticoagulants to reduce the risk of blood clots.
Heart Failure Medications:
Angiotensin-Converting Enzyme Inhibitors: This includes enalapril, lisinopril.
Angiotensin II Receptor Blockers: This includes losartan, valsartan.
Beta-Blockers: This includes carvedilol and metoprolol.
Inotropic Agents:
Milrinone & dobutamine: It is used to give the additional support to heart specifically during the acute cases of the heart failure.
Medication

A left ventricular assist device is a mechanical pump that is surgically inserted into the patient’s chest to help the heart pump blood. People with severe heart failure, whose hearts are unable to pump blood efficiently on their own are the main users of this medical equipment. For those who are not suitable for heart transplantation, LVADs are frequently seen as a bridge to heart transplantation or in some circumstances as destination therapy.

Left ventricular assist device
LVAD is primarily responsible to support the left ventricle, which transports the blood saturated with oxygen from the lungs to the other organs of the body. When heart failure or other conditions damage the heart, LVAD helps to keep the blood flow and enhances the cardiac function.
Severe Heart Failure: Patients who have severe heart failure who are not improving with traditional treatments are treated with LVADs.
Bridge to Transplant: By preserving the circulation and enhancing health, LVADs give individuals to wait for the heart transplantation with short-term support.
Bridge to Recovery: LVADs can be used to help the heart recover from reversible conditions like post-cardiotomy shock or myocarditis.
Post-Cardiotomy Shock: After heart surgery, LVADs help patients who are experiencing circulatory failure.
Inotrope-Dependent Heart Failure: Patients who need intravenous inotropic drugs are evaluated for LVADs.
End-Stage Heart Failure: Patients without mechanical circulatory assistance who have a reduced life expectancy, LVADs can be used for those.
Irreversible end-organ failure: Patients who have severe heart failure who are still eligible for heart transplantation typically undergo assessments for LVADs. The advantages of LVAD support can be limited in patients who have permanent organ failure.
Active infection: If a patient has an active infection, they may be more susceptible to problems with LVAD implementation. Infection can increase the risk of infections associated with devices and cause systemic problems.
Unresolved bleeding disorders: Patients who have coagulation diseases or other bleeding risk may be more susceptible to bleeding problems if they use LVADs.
Severe right ventricular dysfunction: An LVAD can make severe dysfunction in the right ventricular. Other remedies can be useful and taken into consideration in that condition.
Advanced pulmonary disease: LVADs mainly support the left ventricle. It is not effectively address the main cause of pulmonary difficulties. Patient who has severe pulmonary disease may not be suitable for the LVADs.
Pump Unit: The pump is the LVAD’s fundamental component. It supports the heart to pump the blood. There are many types of LVADs like centrifugal and axial flow pumps.
Power Source: LVADs needs an external power source. The patient is connected to an external controller and power pack. Some new device has full implantable system which does not need any external power source.
Controller: The external controller manages and monitors the LVAD’s function. It is a small, portable device that patients carry with them. The controller adjusts the pump speed based on the patient’s activity level and physiological needs.
Power Pack: The power pack is a portable battery that provides power to the LVAD when the patient is away from a direct power source. Patients has spare batteries to make sure the continuous power supply.
Drive Line: The drive line is a cable that connects the implanted pump to the external components, i.e., the controller and power pack. It passes through the patient’s skin, to avoid infections, adequate upkeep and care are essential.
Surgical Driveline Exit Site: This is the area where the drive line exits the patient’s body. It requires regular cleaning and dressing changes to minimize the risk of infection.
It is essential to obtain a complete medical history like previous cardiac events, surgical operations, and pre-existing health issues. The examination method includes determining the severity of heart failure, assessing ventricular function, and finding any further cardiovascular issues. It is essential to make sure that the patient is provided suitable pharmacological drugs to improve the cardiac function and reduce challenges during and after surgery. Formulate an anticoagulant management plan is necessary to prevent thrombus development in the device. It is necessary to provide detailed information on the Left Ventricular Assist Device (LVAD) like the primary purpose, potential side effects, and the need of maintaining the postoperative care guidelines.
It is important that the patient and their family be aware of both the risks and the benefits of the LVAD installation. Before the surgery, efforts must be made to improve the patient’s physiological status. To reduce perioperative risks, it is important to manage comorbidities like diabetes and hypertension.
Pulsatile Flow LVADs: These devices mimic the natural pulsatile motion of heart. It creates a rhythmic activity with each beat. Earlier types of LVADs usually used pulsatile flow.
Continuous Flow LVADs: A large percentage of modern LVADs use continuous flow technology. The blood is continuously pumped by the device. These devices are more compact, durable, and have fewer moving parts that reduces issues.
Axial flow LVADs: It has a single impeller that spins in line with the blood flow axis. These devices have a more streamlined construction and simpler design than other varieties.
Centrifugal Flow LVAD: It uses a spinning impeller to move blood away from the center of rotation. They are known to improve the blood flow and reduce the risk of thrombus development.
Implantable LVADs: These devices are fully implanted into the body, with a driveline which connect the equipment to an external power source and control unit. It is used for long support.
Biventricular help Devices: Other LVADs gives support to the left ventricle. BiVADs gives support to both left and right ventricles. It is used in cases where both chambers fail.
Step 1 The widely recognized method for implementing a durable LVAD entails conducting the implantation while the heart is in operation, utilizing cardiopulmonary bypass.
Step 2 The surgeries involving the implants are conducted using a non-beating heart, cardioplegia & aortic cross-clamp. Additionally, procedures have been carried out both with & without utilization of cardiopulmonary bypass, employing fibrillatory arrest when necessary.
Step 3 Two similar versions of the necessary steps—cutting than sewing or sewing then cutting—can be used to secure the inflow cannula to the left ventricular apex.
Step 4: Using a method of cutting and then sewing, the left ventricular apex undergoes coring. Both procedures are conducted following the administration of suitable anticoagulation.
Step 5 The preference of implanters & the integrity of a heart muscle quality will determine whether to use pledgetted sutures or, interrupted sutures, or running sutures to bind an inflow graft to the left ventricular apex.
Step 6 In the method of sewing before cutting, the inflow graft is firmly attached to the left ventricular apex using the preferred methods & sutures and, subsequently, cored the left ventricular apex.
Step 7 After completing the two processes and excising the left ventricular wall and any papillary muscle from the inflow, the pump is attached to the inflow-sewing ring and securely fastened using the appropriate technique for the pump approach.
Step 8: The aorta is connected to the outflow graft. Usually linked to the ascending aorta on the greater curvature, this is located slightly away from the junction of the sinotubule.
Step 10 The connection can frequently be established using a partial occlusion clamp following the administration of suitable anticoagulants.
Step 11 After a sharp aortotomy, the tiny gauge (5-0,4-0) monofilament suture nonabsorbable is used to sew an outflow graft. Before tying, it is generally beneficial to tighten the suture with nerve hooks.
Step 12 For individuals with an ascending aorta that is calcified rich or those opting for another method, low invasive implant methods, it is possible to attach the outflow graft to a descending thoracic aorta.
Step 13 Using a method like an apical-aortic conduit, centrifugal pumps seem to offer greater adaptability for other implantation strategies.
Step 14 A usual tunnel to the mid-clavicular line is located two to three finger breaths beneath a coastal margin, carrying drivelines that supply power, control, pump & communication.
Step 15 The dermal layer is brought back together, & the driveline is fastened using monofilament suture, which is intended to stay in place till maximum ingrowth occurs in the driveline coating, usually made of velour.
Step 16 With growing frequency, there is a rising trend in adopting alternative implant techniques. These methods may be employed to reduce surgical stress or in response to a challenging mediastinum in individuals undergoing multiple surgical processes.
Complete Blood Count: This test helps monitor for anemia, leukopenia, and thrombocytopenia, which are potential complications associated with LVADs.
Electrolyte Panel: Sodium, Potassium, Chloride: Monitoring electrolyte balance is crucial, especially considering the impact of medications and diuretics on these levels.
Inflammatory Markers: Increased level of C-reactive protein, erythrocyte sedimentation rate may indicate the inflammation or infection that affect the LVAD function.
Hemorrhage: Patients who used LVADs need to administer anticoagulant therapy to mitigate the formation of thrombi which can lead to increased propensity for hemorrhagic incidents. This can lead to gastrointestinal hemorrhage, intracranial hemorrhage, or bleeding at the surgical incision sites.
Infectious Complications: The potential for infections exists at both the implantation site of the LVAD and in the device itself. Sepsis is one of the worst side effects that these diseases can cause.
Thromboembolic Events: Even when anticoagulation procedures are followed, thrombi may still form in the LVAD. This can lead to cerebrovascular accidents or device malfunction.
Mechanical Dysfunction: Mechanical devices are prone to operational failures because of their intrinsic nature. This can lead to complications like electrical or pump breakdowns. To identify and address these problems as soon as possible, routine observation is essential.
Right Ventricular Insufficiency: While LVADs mainly support left ventricular function, there is some possibility for the right heart to deteriorate over time. This condition may lead to failure of right ventricular which needs medical interventions.
Arrhythmogenic Events: The presence of LVADs may cause arrhythmias. Such arrhythmias in people with LVADs may cause significant clinical problems.
Warfarin: It helps to prevent the blood clots. Regularly monitor the international normalized ratio is needed.
Antiplatelet Agents:
Aspirin: Often used in conjunction with anticoagulants to reduce the risk of blood clots.
Heart Failure Medications:
Angiotensin-Converting Enzyme Inhibitors: This includes enalapril, lisinopril.
Angiotensin II Receptor Blockers: This includes losartan, valsartan.
Beta-Blockers: This includes carvedilol and metoprolol.
Inotropic Agents:
Milrinone & dobutamine: It is used to give the additional support to heart specifically during the acute cases of the heart failure.

Both our subscription plans include Free CME/CPD AMA PRA Category 1 credits.

On course completion, you will receive a full-sized presentation quality digital certificate.
A dynamic medical simulation platform designed to train healthcare professionals and students to effectively run code situations through an immersive hands-on experience in a live, interactive 3D environment.

When you have your licenses, certificates and CMEs in one place, it's easier to track your career growth. You can easily share these with hospitals as well, using your medtigo app.



