Background
Wound healing is a restorative process that starts with superficial skin injury and progresses to a complex cascade of cellular functions that rebuild tissues with their original tensile strength and resurface.
Healing is a systematic process involving four classic phases: clot formation, inflammation, cell proliferation and ultimately cell maturation. Platelets are the key in hemostasis, inflammatory cells debris, proliferative involve epithelization, fibroplasia angiogenesis, granulation tissue forms and maturation phase increases scar strength.
The mechanism of how wound repair takes place is a complicated one, consisting of interrelated cellular events that subsequently lead to scarring but not all aspects of this process are yet fully understood.
The initial hemostasis is triggered by platelets clotting process and release of cytokines, chemokines, or hormones. Vasoconstriction, by the action of vasoactive tackles limiting blood loss to prevent wound blanching for a time.
The damaged intima layer, containing collagen tissue and tissue factor, promotes platelet aggregation. Consequently, there is platelet clumping and a clot forms. As a result, there is the release of chemotactic and growth factors that draw the inflammatory cells and startup the healing process.
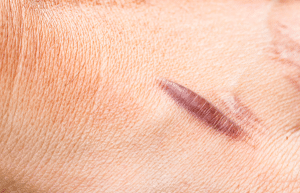
Hypertrophic scarring
Indications
Contraindications
Outcomes
Inflammatory Phase
Thrombin generation by clotting casc
ades permits plasma leakage, this in turn aids in the move of inflammatory cells to the site of injury, inhibits the clot formation process and then turns plasmin into a cell lysis enzyme.
The initial vasoconstriction stage lasts for 10-15 minutes, after which vasodilation continues as a consequence reaction to histamine; prostaglandins and kinins, as well as leukotrienes. As such more blood flow, white cells of inflammation and infectious factors accumulate in the wound area leading to pain.
In mere hours, an inflammatory phase, that primarily consists of the active recruitment and engulfment of infectious microbes and dead cells via neutrophils and macrophages, and lymphocytes, takes places, albeit not required for wound healing. Neutrophils do purge the wound sites from bacteria and necrotized matters, release inflammatory mediators as well, and bactericidal oxygen free radicals, their absence does not halt wounds processing.
Macrophages playing a leading role in healing wound process phagocytosing blood clots, secreting collagenases and elastases, and releasing PDGF. These as well exert the actions of fibroblast proliferation and angiogenesis. Growth factors accumulated by macrophage are critical to restore a new tissue, manifested the appearance of defects in new tissue formation of macrophage-depleted wounds on animals. The lymphocytes of the T cell migrate in the wound 72 hours after injury, via the chemokines such as IL-1, lymphokines and antibodies creation.
Proliferative Phase
Granulation tissue is characterized by the existence of inflammatory cells, fibroblasts, and neovascular network in a matrix of fibronectin, collagen, glycosaminoglycans, and proteoglycans. It emerges at the end of the third to the fifth day of the injury.
Epithelialization
Epithelization is the process of forming a protective barrier of wound edge or underlying wound in 24-48 hours post wounding which includes migration and growth of the cells.
The reepithelialization process is triggered already within the wound’s first hours, carding off the epidermal cells from the edges of the wound, and inducing the formation of intracellular actin microfilaments. In cases of the surface damage the adnexal structures aid this process.
Skin cells degrade collagen, and plasminogen activators bring on plasmin production that eventually causes clot dissolution. Migrating cells Affairs with fibrin matrix Fibronectin preserves keratinocyte adhesion. Injuries in moist conditions promote epithelialization fast, and occlusive dressings balance the humidity in the extracellular matrix leading to faster epithelial regeneration. Consequently, the inflammatory agents will be limited and the skin will be hydrated especially during the first 48 hours.
Fibroplasia
Fibroblasts start growing, while the inflammatory cells decrease, since the chemotactic factors and inactivated inflammatory cells no longer get them produced and inactivated.
Fibroplasia, which is a pathological phenomenon where components of the skin fibroblasts and mesenchymal cells proliferate math is a significant event that correlates with a period of fibronectin for 3 to 5 days.
Collagen mass synthesis and plasticity are among the significant processes that occur during the proliferative stage of wound healing. The critical role of oxygen, vitamin C, ferrous iron, and α-ketoglutarate in the hydroxylation of the collagen, which results in insufficient hydroxylation when there is deficiency in the collagen formation. Collagen extracted in procollagen form is cleaved to tropocollagen which further aggregates with other molecules to get into collagen filaments. Fibroblasts produce a matrix gel of glycosaminoglycans including hyaluronic acid, chondroitin sulfate, dermatan sulfate, and heparin sulfate.
Elastin, which is a structural protein with a random coil formation contributes to the skin stretch and recoil properties that are crucial for the restoration of the original shape of the damaged area.
Angiogenesis
Nourishing and sometimes darkening the growing tissue that mainly affects scar erythema is performed through the phenomenon of new blood supply and circulatory system. The same macrophages encourage angiogenesis by releasing angiogenic factors: fibroblast growth factor, and vascular endothelial factor that attracts and bonds with surrounding endothelial cells.
Angiogenesis enhances blood flow to wounds, promoting healing factors. Nevertheless, this is transient phenomena, since vascular demand does not remain unchanged, growing and getting stronger, until the process of apoptosis takes place and unnecessary vessels disappear.
Contraction
The wound contraction that has collagen synthesis as the first step and reaches its peak by the 5-15th day post wounding is the process which closes wound defects. The healing takes place by contracting the wound area at the rate of 0.75 mm/d, which depends on the tissue laxity and the shape of the wound. HSC activation also depends on myofibroblasts and extracellular matrix components.
Maturation Phase
Collagen
Collagen remodeling during maturation remodeling, synthesis factors like collagenases and matrix metalloproteinases can not only aiding in collagen debris but also in tissue repair. Tissue inhibitors are the limiting factor for these enzymes, thus they will prevent more than enough collagen being formed as well as the old collagen being removed.
The process of healing response is through the method of collagen organization, fibrin replacement, the conversion of the type III collagen type with the type I, and the dissolving of water from the scar.
Cytokines
Cytokines, proteins released from the cells, act as fundamental principles of wound healing because they bind surface receptors to various routes via cell signalling.
Primary and Secondary Healing
The primary healing is defined as a non-infected, non-delayed well-approximated surgery, which includes the respect of the forth of the four phases, no interruption.
Secondary intention healing on the other hand proceeds mostly by granulation tissue generation and epithelialization, a process that is slower compared to primary intention healing because it takes longer times for new blood vessels to form and for epithelialization to occur. This makes wounds prone to infection and poor healing.
Abnormal Wound Healing
Keloids and hypertrophic scars are two different types of scars, with an increase in collagen supply and a decrease in its lytic breakdown being the main common characteristics. A keloid is a scar that goes beyond the scar border, while a hypertrophic scar is a scar that recedes in the direction of the joint and is associated with joint contractures.
Histological examination will reveal keloids and hypertrophic scars, with the former having a prominent blood supply, high mesenchymal density, and thick epidermis, and the latter having unorganized collagen fibers. Type III collagen is the main component of hypertrophic scars.
The mechanisms of Keloid and hypertrophic scar formation are interrelated with the abnormalities in cell movements, proliferation, inflammation, extracellular matrix proteins, cytokines, and remodeling. Transforming growth factor-β acts as a fundamental link in the physiologic processes associated with pathologic healing.
Both hypertrophic scars and keloids are observed much more frequently in dark-skinned individuals, across different skin tension lines as well as on ear piercings.
Diabetes and Wound Healing
Microangiopatic disease which progresses to whole blood flow impairment, peripheral neuropathy, tissue trauma, infection risk, weakened immune function, slow collagen synthesis and failure to heal tenerly, leads to the destruction of blood vessels and maylet to the works dehiscence.
Controlling blood glucose levels is one of the key factors in the wound healing of diabetics with more than 200 mg/dL making worse outcomes.
Effects of Smoking on Wound Healing
Smoking is detrimental to repairing by hampering phagocytic process, disrupting collagen synthesis, and raising carboxy-hemoglobin levels resulting from the direct toxic effects and vasoconstriction of nicotine.
These results suggest that smoking delays inflammatory processes, as the eyes fluid found in the drainage systems of smokers was depleted of epidermal growth factor and FMS-like tyrosine kinase-1, compared with non-smokers.
Surgeons recommend 4-6 week periods of abstinence from smoking both before and after cosmetic surgeries, because smoking cessation somehow balance metalloproteinase levels and the number of type I collagen, and other factors that can results in poor wound healing.

Wound healing is a restorative process that starts with superficial skin injury and progresses to a complex cascade of cellular functions that rebuild tissues with their original tensile strength and resurface.
Healing is a systematic process involving four classic phases: clot formation, inflammation, cell proliferation and ultimately cell maturation. Platelets are the key in hemostasis, inflammatory cells debris, proliferative involve epithelization, fibroplasia angiogenesis, granulation tissue forms and maturation phase increases scar strength.
The mechanism of how wound repair takes place is a complicated one, consisting of interrelated cellular events that subsequently lead to scarring but not all aspects of this process are yet fully understood.
The initial hemostasis is triggered by platelets clotting process and release of cytokines, chemokines, or hormones. Vasoconstriction, by the action of vasoactive tackles limiting blood loss to prevent wound blanching for a time.
The damaged intima layer, containing collagen tissue and tissue factor, promotes platelet aggregation. Consequently, there is platelet clumping and a clot forms. As a result, there is the release of chemotactic and growth factors that draw the inflammatory cells and startup the healing process.

Hypertrophic scarring
Thrombin generation by clotting casc
ades permits plasma leakage, this in turn aids in the move of inflammatory cells to the site of injury, inhibits the clot formation process and then turns plasmin into a cell lysis enzyme.
The initial vasoconstriction stage lasts for 10-15 minutes, after which vasodilation continues as a consequence reaction to histamine; prostaglandins and kinins, as well as leukotrienes. As such more blood flow, white cells of inflammation and infectious factors accumulate in the wound area leading to pain.
In mere hours, an inflammatory phase, that primarily consists of the active recruitment and engulfment of infectious microbes and dead cells via neutrophils and macrophages, and lymphocytes, takes places, albeit not required for wound healing. Neutrophils do purge the wound sites from bacteria and necrotized matters, release inflammatory mediators as well, and bactericidal oxygen free radicals, their absence does not halt wounds processing.
Macrophages playing a leading role in healing wound process phagocytosing blood clots, secreting collagenases and elastases, and releasing PDGF. These as well exert the actions of fibroblast proliferation and angiogenesis. Growth factors accumulated by macrophage are critical to restore a new tissue, manifested the appearance of defects in new tissue formation of macrophage-depleted wounds on animals. The lymphocytes of the T cell migrate in the wound 72 hours after injury, via the chemokines such as IL-1, lymphokines and antibodies creation.
Granulation tissue is characterized by the existence of inflammatory cells, fibroblasts, and neovascular network in a matrix of fibronectin, collagen, glycosaminoglycans, and proteoglycans. It emerges at the end of the third to the fifth day of the injury.
Epithelialization
Epithelization is the process of forming a protective barrier of wound edge or underlying wound in 24-48 hours post wounding which includes migration and growth of the cells.
The reepithelialization process is triggered already within the wound’s first hours, carding off the epidermal cells from the edges of the wound, and inducing the formation of intracellular actin microfilaments. In cases of the surface damage the adnexal structures aid this process.
Skin cells degrade collagen, and plasminogen activators bring on plasmin production that eventually causes clot dissolution. Migrating cells Affairs with fibrin matrix Fibronectin preserves keratinocyte adhesion. Injuries in moist conditions promote epithelialization fast, and occlusive dressings balance the humidity in the extracellular matrix leading to faster epithelial regeneration. Consequently, the inflammatory agents will be limited and the skin will be hydrated especially during the first 48 hours.
Fibroplasia
Fibroblasts start growing, while the inflammatory cells decrease, since the chemotactic factors and inactivated inflammatory cells no longer get them produced and inactivated.
Fibroplasia, which is a pathological phenomenon where components of the skin fibroblasts and mesenchymal cells proliferate math is a significant event that correlates with a period of fibronectin for 3 to 5 days.
Collagen mass synthesis and plasticity are among the significant processes that occur during the proliferative stage of wound healing. The critical role of oxygen, vitamin C, ferrous iron, and α-ketoglutarate in the hydroxylation of the collagen, which results in insufficient hydroxylation when there is deficiency in the collagen formation. Collagen extracted in procollagen form is cleaved to tropocollagen which further aggregates with other molecules to get into collagen filaments. Fibroblasts produce a matrix gel of glycosaminoglycans including hyaluronic acid, chondroitin sulfate, dermatan sulfate, and heparin sulfate.
Elastin, which is a structural protein with a random coil formation contributes to the skin stretch and recoil properties that are crucial for the restoration of the original shape of the damaged area.
Angiogenesis
Nourishing and sometimes darkening the growing tissue that mainly affects scar erythema is performed through the phenomenon of new blood supply and circulatory system. The same macrophages encourage angiogenesis by releasing angiogenic factors: fibroblast growth factor, and vascular endothelial factor that attracts and bonds with surrounding endothelial cells.
Angiogenesis enhances blood flow to wounds, promoting healing factors. Nevertheless, this is transient phenomena, since vascular demand does not remain unchanged, growing and getting stronger, until the process of apoptosis takes place and unnecessary vessels disappear.
Contraction
The wound contraction that has collagen synthesis as the first step and reaches its peak by the 5-15th day post wounding is the process which closes wound defects. The healing takes place by contracting the wound area at the rate of 0.75 mm/d, which depends on the tissue laxity and the shape of the wound. HSC activation also depends on myofibroblasts and extracellular matrix components.
Collagen
Collagen remodeling during maturation remodeling, synthesis factors like collagenases and matrix metalloproteinases can not only aiding in collagen debris but also in tissue repair. Tissue inhibitors are the limiting factor for these enzymes, thus they will prevent more than enough collagen being formed as well as the old collagen being removed.
The process of healing response is through the method of collagen organization, fibrin replacement, the conversion of the type III collagen type with the type I, and the dissolving of water from the scar.
Cytokines
Cytokines, proteins released from the cells, act as fundamental principles of wound healing because they bind surface receptors to various routes via cell signalling.
The primary healing is defined as a non-infected, non-delayed well-approximated surgery, which includes the respect of the forth of the four phases, no interruption.
Secondary intention healing on the other hand proceeds mostly by granulation tissue generation and epithelialization, a process that is slower compared to primary intention healing because it takes longer times for new blood vessels to form and for epithelialization to occur. This makes wounds prone to infection and poor healing.
Keloids and hypertrophic scars are two different types of scars, with an increase in collagen supply and a decrease in its lytic breakdown being the main common characteristics. A keloid is a scar that goes beyond the scar border, while a hypertrophic scar is a scar that recedes in the direction of the joint and is associated with joint contractures.
Histological examination will reveal keloids and hypertrophic scars, with the former having a prominent blood supply, high mesenchymal density, and thick epidermis, and the latter having unorganized collagen fibers. Type III collagen is the main component of hypertrophic scars.
The mechanisms of Keloid and hypertrophic scar formation are interrelated with the abnormalities in cell movements, proliferation, inflammation, extracellular matrix proteins, cytokines, and remodeling. Transforming growth factor-β acts as a fundamental link in the physiologic processes associated with pathologic healing.
Both hypertrophic scars and keloids are observed much more frequently in dark-skinned individuals, across different skin tension lines as well as on ear piercings.
Microangiopatic disease which progresses to whole blood flow impairment, peripheral neuropathy, tissue trauma, infection risk, weakened immune function, slow collagen synthesis and failure to heal tenerly, leads to the destruction of blood vessels and maylet to the works dehiscence.
Controlling blood glucose levels is one of the key factors in the wound healing of diabetics with more than 200 mg/dL making worse outcomes.
Smoking is detrimental to repairing by hampering phagocytic process, disrupting collagen synthesis, and raising carboxy-hemoglobin levels resulting from the direct toxic effects and vasoconstriction of nicotine.
These results suggest that smoking delays inflammatory processes, as the eyes fluid found in the drainage systems of smokers was depleted of epidermal growth factor and FMS-like tyrosine kinase-1, compared with non-smokers.
Surgeons recommend 4-6 week periods of abstinence from smoking both before and after cosmetic surgeries, because smoking cessation somehow balance metalloproteinase levels and the number of type I collagen, and other factors that can results in poor wound healing.

Both our subscription plans include Free CME/CPD AMA PRA Category 1 credits.

On course completion, you will receive a full-sized presentation quality digital certificate.
A dynamic medical simulation platform designed to train healthcare professionals and students to effectively run code situations through an immersive hands-on experience in a live, interactive 3D environment.

When you have your licenses, certificates and CMEs in one place, it's easier to track your career growth. You can easily share these with hospitals as well, using your medtigo app.



